ResMed Urgent Field Safety Notice
ResMed has issued an Urgent Field Safety Notice to advise that they are updating the 'Contraindications' and 'Warnings' of selected masks and mask components. Please note, this recall is a correction, not a product removal, it is only an update of the information provided in the user guides of masks with magnets manufactured by Resmed.
Resmed has provided patients with the following resources:
Please read the Patient Letter and its list of contraindications carefully, and evaluate whether you are affected.
Find out if you are affected:
If you do not use a mask with magnets, the updated contraindications and warnings do not apply to you.
The updated contraindications for ResMed masks with magnets only apply if you, or anyone in close physical contact with you while using the mask (e.g., bed partner), has a certain implant or medical device that is identified in the notice.
If you use a ResMed mask with magnets, you should also be aware of the updated warning in the notice.
If you made this purchase for someone else, please notify the recipient.
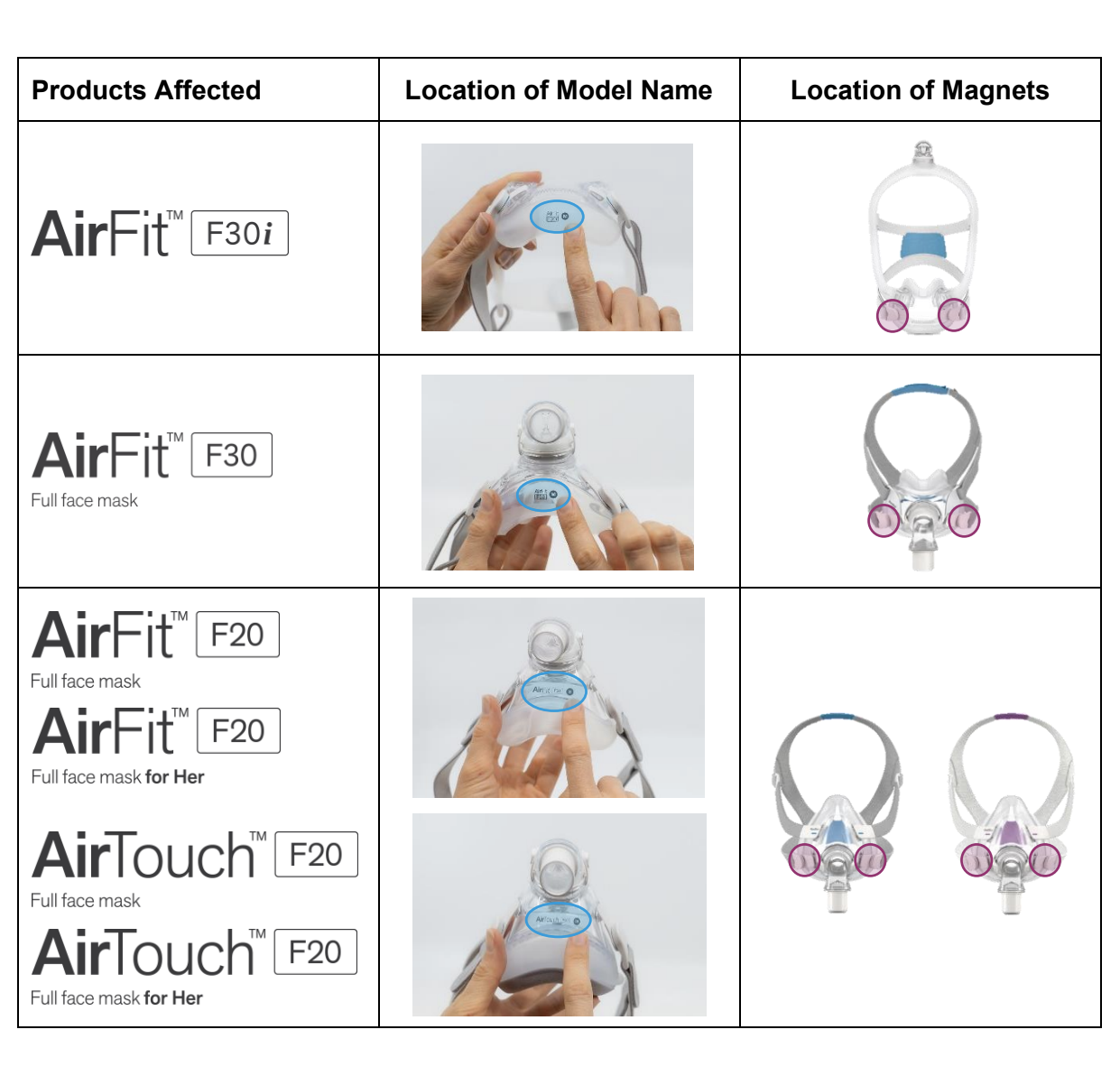
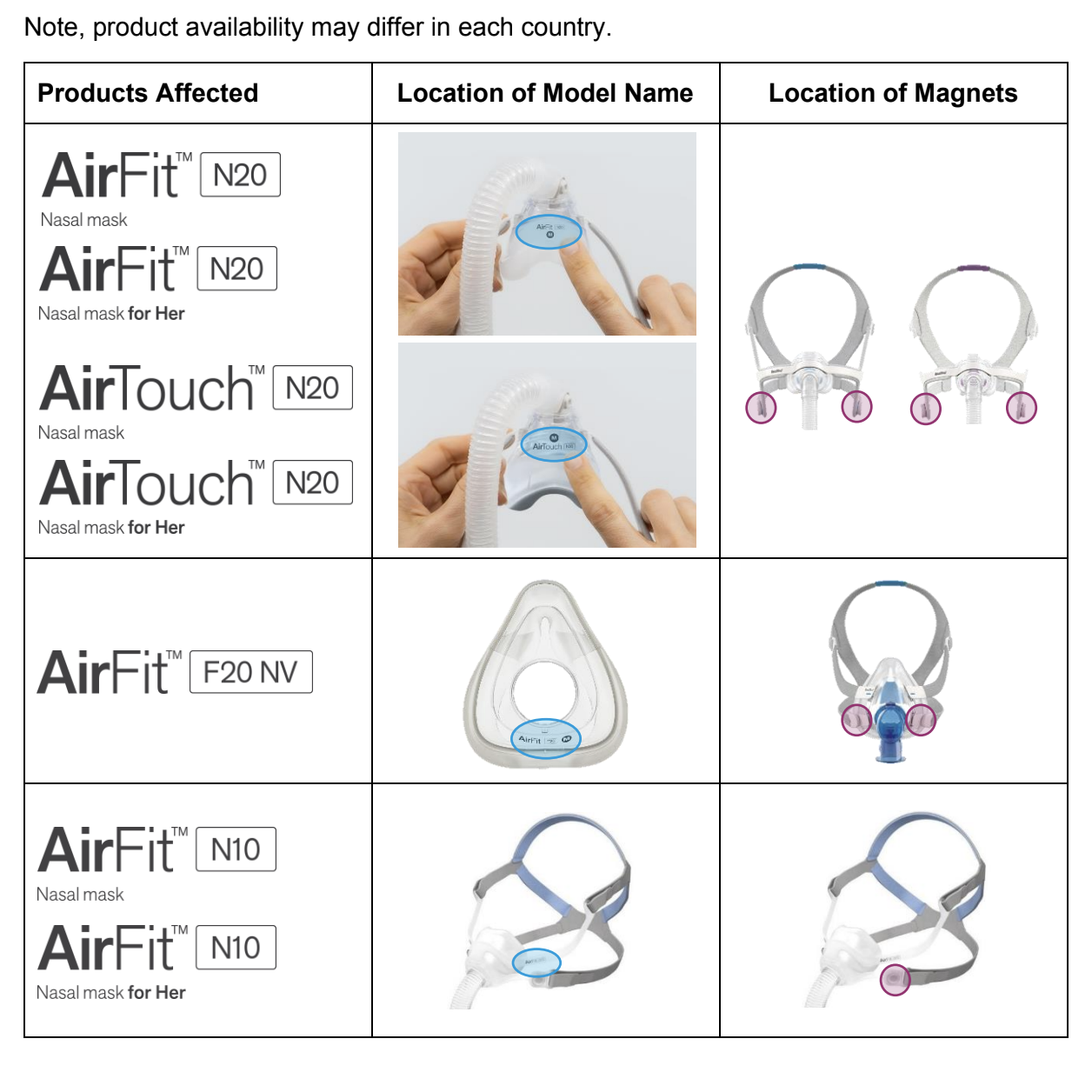
The linked resources provide a full list of impacted products and how to identify them. Impacted masks include:
FAQs for Mask users
-
ResMed’s masks with magnets are safe when used in accordance with the updated instructions for use, including the contraindications and warnings.
-
Magnets are used in some ResMed masks to provide a simple and easy way for the user to attach and detach the headgear to the mask frame. This can be notably beneficial to users with disabilities, including those facing dexterity or vision impairment.
-
Ferromagnetic refers to a material property that is influenced by magnetic fields (e.g., attracts/repels under magnetic fields). Materials that are ferromagnetic are generally made of iron, cobalt, nickel, or some alloys made of these elements.
Not all medical implants listed in the contraindications are made from ferromagnetic materials. If you are not sure whether an implant or medical device falls under the contraindications, or you require additional information on the potential adverse effects of magnetic fields for your particular implant or medical device, please contact your physician/doctor.
-
Replace your mask containing magnets with an alternative mask without magnets in a timely manner. Contact us for alternative mask options.
Note, ResMed is making our masks without magnets available to your mask provider for replacement purposes for contraindicated patients. If an alternative mask is not available, consult your physician/doctor for appropriate next steps regarding your therapy.
If you are not sure whether an implant or medical device falls under the contraindications, or you require additional information on the potential adverse effects of magnetic fields for your particular implant or medical device, please contact us or physician/doctor. -
Contact us for alternative non-magnet mask options. Note, ResMed is making our masks without magnets available to your mask provider for replacement purposes for contraindicated patients.